Australia’s CSL Prices Hemophilia Gene Therapy at $3.5M
Australian drugmaker CSL Ltd (CSL.AX), opens new tab on Tuesday set the list price of its one-time gene therapy for hemophilia B at $3.5…

Australian drugmaker CSL Ltd (CSL.AX), opens new tab on Tuesday set the list price of its one-time gene therapy for hemophilia B at $3.5…

After a prolonged journey, the medicine, known as Roctavian, is now cleared for certain patients with hemophilia A, the more common form of the…

The Food and Drug Administration on Friday granted accelerated approval to a personalized gene therapy for an ultra-rare childhood brain disease, called cerebral adrenoleukodystrophy…
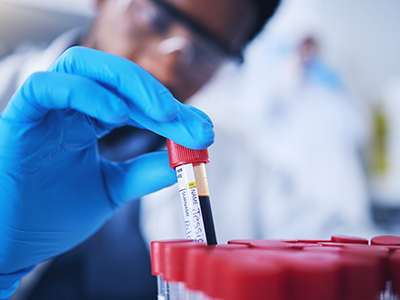
The U.S. Food and Drug Administration on Wednesday approved bluebird bio's (BLUE.O), opens new tab gene therapy for patients with a rare disorder requiring…

Following back-to-back approvals in lymphocytic leukemia, Bristol Myers Squibb’s CAR-T therapy Breyanzi on Wednesday won the FDA’s green light for relapsed or refractory follicular…
In the years since gaining an initial FDA approval for Breyanzi, Bristol Myers Squibb has worked hard to expand the reach of its cell…

The FDA has cleared Bristol Myers’ Breyanzi to treat newly relapsed or refractory large B-cell lymphoma (LBCL). The nod comes less than three months after Yescarta became first…

False Claims Act Settlements and Judgments Exceed $2.68 Billion in Fiscal Year 2023 - Highest Number of Settlements and Judgements in History

Aucatzyl is a CAR T-cell therapy that targets CD19 and has been designed to minimize excessive activation of the programmed T cells. The wholesale…